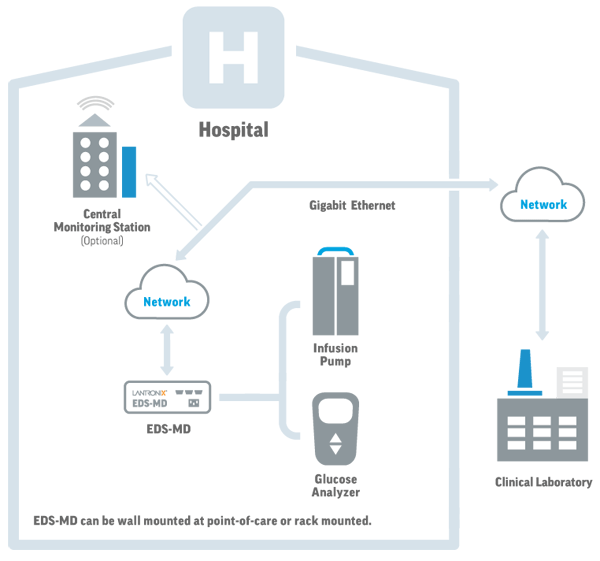
Description
Overview for EDS-MD
Manage Visibility into Medical Assets
The medical industry utilizes many connected devices and assets. A majority of these devices are critical to the operation of the medical enterprise, for example, patient monitors. The EDS-MD can be integrated into the medical enterprise’ network and enable visibility into these devices from anywhere, anytime.
Manage Mission-Critical Data Securely
The transport of sensitive information to and from remote device servers over the Internet demands the highest level of security. With Lantronix’ proprietary Secure Com Port Redirector™ virtualization software, existing serial port applications can work with EDS-MD without any code modifications. Virtual COM ports can be mapped to EDS-MD and encrypted at both ends of communication.
Manage Assets and Data Locally or Remotely
Management of any device and data requires fault-tolerance. EDS-MD empowers authorized IT staff to access and monitor devices and data anywhere, anytime. In addition, the EDS-MD goes beyond the IEC-60601 requirement, as it provides galvanic isolation on all ports to ensure the highest levels of safety and fault-tolerance. Should a grounding issue ever occurs, the operation of EDS-MD or other connected devices will not be affected.
High-Performance Wired Connectivity
The EDS-MD offers 4, 8 or 16 individually isolated RS-232 serial ports with a Gigabit (10/100/1000Mpbs) network interface. The configuration and setup of the EDS-MD is simple with industry-standard management tools including integrated Web and CLI interfaces along with XML support. For best overall operation, it can be managed by Lantronix Gateway Central powered by MACH 10.
Specifically designed for the medical industry, the UL and IEC-60601 compliant EDS-MD provides mission-critical device connectivity, allowing remote access and management of virtually any electronic or medical device.EDS-MD offers the benefit of 4, 8 or 16 individually isolated RS-232 serial ports with a Gigabit (10/100/1000Mpbs) network interface. Going above and beyond the IEC-60601 requirement, EDS-MD provides galvanic isolation on all ports to ensure the highest levels of safety. If a grounding problem occurs, the operation of EDS-MD or other connected devices will not be affected.
With Lantronix’ proprietary Secure Com Port Redirector virtualization software, existing serial port applications can work with EDS-MD without any code modifications. Virtual COM ports can be mapped to EDS-MD and encrypted at both ends of the communication, allowing the transport of sensitive information to and from remote device servers over the Internet with the highest level of security.
Set up and configuration of EDS-MD is simple with industry-standard management tools including Web, CLI, XML and Lantronix Gateway Central powered by MACH10.
EDS-MD – Certified Multi-Port Wired IoT Gateway for Medical Devices